Battery Day
Tesla had its first Battery Day on September 22nd, 20201. What a fantastic world we live in that we can witness the first Apple-like keynote for batteries. Batteries are a part of our everyday life; without them, the world would be a much different place. Your cellphone, flashlight, tablet, laptops, drones, cars, and other devices would not be portable and operational without batteries.
At the heart of it, batteries store chemical energy and convert it into electrical energy. The chemical reaction in a battery involves the flow of electrons from one electrode to another. When a battery is discharging, electrons flow from the electrode known as the anode, or negative electrode, to the electrode known as the cathode, or positive electrode. This flow of electrons provides an electric current that can be used to power devices. Electrons have a negative charge; therefore, as the flow of negative electrons moves from one electrode to another, an electrolyte is used to balance the charge by being the route for charge-balancing positive ions to flow.
Let’s break this process down a bit and uncover the chemical reactions at play within batteries. To have an electrical current, we need a flow of electrons. Where do those electrons come from?
Electrons in the anode are produced by a chemical reaction between the anode, or negative electrode, and the electrolyte. Simultaneously, another chemical reaction occurs in the cathode, or positive electrode, enabling it to accept electrons. Through these chemical reactions, a flow of electrons is created, resulting in an electrical current.
A chemical reaction that involves the exchange of electrons is known as a reduction-oxidation reaction, or redox reaction. Reduction refers to a gain of electrons. Thus, half of this reaction, defined as reduction, occurs at the cathode because it gains electrons. Oxidation refers to a loss of electrons. Therefore, half of this reaction, defined as oxidation, occurs at the anode because it loses electrons to the cathode. Each of these reactions, reduction and oxidation, has a particular standard potential. An electrochemical cell can be made up of any two conducting materials that have reactions with different standard potentials since the more robust material, which makes up the cathode, will gain electrons from the weaker material, which makes up the anode.
Batteries can be made up of one or more electrochemical cells, each cell consisting of one anode, one cathode, and an electrolyte, as described above. The electrodes and electrolyte are generally made up of different types of metals or other chemical compounds. Different materials for the electrodes and electrolyte produce different chemical reactions that affect how the battery works, how much energy it can store, and its voltage.
Volts
The word “volt” refers to the measure of electric potential. The term came from the Italian scientist Alessandro Volta, who is credited for inventing the first battery. In 1780, Luigi Galvani, another Italian scientist, observed that the legs of frogs hanging on iron or brass hooks would twitch when touched with a probe of some other type of metal. Galvani believed that this was caused by electricity from within the frogs’ tissues. He called it ‘animal electricity.’
Volta believed the electric current came from the two different metal types: the hooks on which the frogs were hanging and the probe’s different metal. He thought the current was merely being transmitted through, not from, the frogs’ tissues. Volta experimented with stacks of silver and zinc layers interspersed with layers of cloth or paper soaked in saltwater and found an electric current flowed through a wire applied to both ends of the pile. Volta also found that the amount of voltage could be increased by using different metals in the pile. Leading to what we know today as the scientific unit of a “volt2.”
There are two ways to increase a battery’s voltage: stack several cells together or increase the cell’s electrochemical potential by choosing different materials.
When cells are combined in a series, it has an additive effect on the battery’s voltage. Essentially, the force at which the electrons move through the battery can be seen as the total force as it moves from the first cell’s anode through the number of cells the battery contains to the last cell’s cathode.
In contrast, when cells are combined in parallel, it increases the battery’s possible current, which is defined as the total number of electrons flowing through the cells, but not its voltage.
Measuring electricity
When you buy a light bulb, the box indicates the wattage for the bulb. Watts are a measurement of power. Watts describe the rate of electricity that is being used at a specific moment. Therefore, a 60-watt light bulb uses 60 watts of electricity at any moment while turned on.
Watt-hours (Wh), on the other hand, are a measurement of energy. Watt-hours describe the total amount of electricity used over time. You can derive from the name that watt-hours are a combination of watts, the rate electricity is used, and hours, the length of time used. Going back to our example, a 60-watt light bulb that draws 60 watts of electricity at any moment while turned on uses 60 watt-hours of electricity over one hour.
Watt-hours will only get you so far, however. If you want to measure the electricity used by a large appliance or a household, folks tend to use kilowatt-hours (kWh). A kilowatt is equal to one thousand watts; therefore, one kilowatt-hour is equal to one thousand watt-hours.
If you want to measure the output of a power plant or the amount of electricity used by an entire city, you will use megawatts. A megawatt is one thousand kilowatts or one million watts. Getting even larger, a gigawatt is one thousand megawatts, or one million kilowatts, or one billion watts. Gigawatts is where the namesake for Tesla’s Gigafactories comes from. In 2018, battery production at the Gigafactory in Nevada reached 20 gigawatt-hours (GWh) per year3.
Alkaline batteries
Most people are probably familiar with alkaline batteries. These are the batteries that you typically use to power toys, electronics, flashlights, etc. The bulk of alkaline batteries produced are single-use, although there are some rechargeable alkaline batteries in existence. So what makes up an alkaline battery?
Alkaline batteries have zinc as their anode and manganese dioxide (MnO2) as their cathode. Their name, however, comes from the alkaline solution used as the electrolyte. The electrolyte is typically potassium hydroxide (KOH), which can contain a large number of dissolved ions. The more ions the electrolyte solution can absorb, the longer the redox reaction that drives the battery can keep going.
The zinc anode is usually in powdered form. Powder has a greater surface area for a reaction, which means the cell can quickly release its power. The zinc anode gives up its electrons to the manganese dioxide cathode, to which carbon, in the form of graphite, is added to improve its conductivity and help it keep its shape.
Alkaline batteries are popular because they have a low self-discharge rate, giving them a long shelf life, and don’t contain toxic heavy metals like lead or cadmium. They account for the bulk of batteries that are made today, although their place at the top will likely soon be challenged by the lithium-ion batteries in our phones, laptops, cars, and an increasing number of other gadgets.
Lithium-ion batteries
Lithium-ion batteries are popular due to their energy density. Because the energy is dense, your phone can last all day and still be the small, portable, handheld device we are all familiar with. As you likely know from the behavior of your phone, lithium-ion batteries are rechargeable. The namesake for the battery comes from the fact that lithium ions (Li+) are involved in the chemical reactions that make up the battery.
In a lithium-ion cell, both electrodes, anode and cathode, are made of materials that can absorb lithium ions. The absorbing action is known as intercalation when charged ions of an element can be stored inside a material without significantly disturbing it. The lithium ions are paired to an electron within the structure of the anode. When the battery discharges, the intercalated lithium ions are released from the anode and travel through the electrolyte solution to be intercalated in the cathode.
A lithium-ion battery starts its life in a state of full discharge: all its lithium ions are intercalated within the cathode, and its chemistry cannot yet produce any electricity. Before the battery can be used, it needs to be charged. As the battery is charged, an oxidation reaction occurs at the cathode, meaning that it loses some negatively charged electrons. An equal number of positively charged intercalated lithium ions are dissolved into the electrolyte solution to maintain the charge balance in the cathode. These travel over to the anode, where they are intercalated, or absorbed, within what is typically graphite. This intercalation reaction also deposits electrons into the graphite anode, to pair with the lithium ion. There are many other types of batteries, but you mostly need to understand lithium-ion batteries as context for this article4.
New technologies
Solid-state batteries
Counter to the liquid or polymer gel electrolyte found in batteries today, solid-state batteries use a solid electrolyte and solid electrodes. If we recall from earlier, positive ions flow through the electrolyte to balance the electrons’ negative charge. Today, batteries are quite efficient at transferring positive ions since a liquid electrolyte is in contact with the electrodes’ entire surface area. Using a solid makes this a bit harder. Imagine the difference between dipping a chip in soup and dipping it into chopped tomatoes. The chip dipped in the soup will have soup covering more of the chip’s surface area than the chopped tomatoes cover the other chip.
So why even use a solid electrolyte if it is less efficient? Today’s lithium-ion batteries typically rely on flammable liquids as the electrolyte. By using a solid electrolyte, batteries can be less prone to catching fire. Most folks probably remember Samsung’s Galaxy Note 7, which had the unfortunate side effect of catching fire5. Solid electrolytes provide a much safer alternative.
Research and experimentation in solid electrolytes typically tend to be either solid polymers at high temperatures or ceramics at room temperature. The downside of solid polymers at high temperatures is they need to operate at temperatures above 220°F (105°C)6. That is certainly not practical for a handheld device like a phone or tablet, but could be apt for storing energy to power a home.
Quite a few companies are working on using ceramics at room temperature to create a solid-state battery. Toyota has been talking about theirs for years7 and aims to have it completed in 20258. Startups, such as Solid Power and A123 Systems (with the help of Iconic Materials), aim to do the same.
A lot of the novel research being done on solid-state batteries is the work of Jürgen Janek9. Jürgen recently published a benchmark of the performance of all-solid-state lithium batteries10. Another high-profile battery scientist, Gerbrand Ceder, published a paper on interface stability in solid-state batteries11. New and novel research on solid-state batteries is being published quite frequently. While there are many skeptics of solid-state batteries since it has yet to be commercially delivered and scaled, I would not dismiss it entirely from having a seat at the table in the future.
Nuclear batteries
Until now, we have only discussed batteries powered by chemical reactions, such as those powering flashlights, phones, and other gadgets. Chemical batteries, also known as galvanic cells, discharge in a given amount of time and either need to be thrown away or recharged. Begging the question: is there a type of battery that could last long term?
Nuclear batteries, also known as atomic batteries, using the energy of beta decay, are being researched to create a battery that lasts longer than those powered by chemical reactions. Batteries powered by beta decay are known as betavoltaics. Radioactive isotopes used in nuclear batteries have half-lives ranging from tens to hundreds of years, so their power output remains nearly constant for a very long time. If nuclear batteries last from tens to hundreds of years, why are we not using them everywhere today? Doesn’t everyone want a phone that could last at least ten years without needing to be charged?
There are a few side effects of nuclear batteries. They cannot be turned off; electrons are continually being produced, even when they are not needed. Research is being done into stimulating beta decay12, which would create more current on-demand, allowing the output to drop to almost nothing when it is turned off. Another downside is the power density of betavoltaic cells is much lower than that of chemical batteries. However, it is interesting to note that betavoltaics were used in the 1970s to power cardiac pacemakers, before being replaced by cheaper lithium-ion batteries, even though lithium-ion batteries have a shorter lifetime.
In 2016, Russian researchers from MISIS presented a prototype betavoltaic battery based on nickel-6313. A downside of using nickel-63 is that it is not readily available, making their research hard to commercialize. CityLabs sells a betavoltaic battery, with a 14.4-year half-life, you can buy today starting at $1,00014, but you would need 1.2 million of these just to have one watt of power. NDB is a startup working on a nano diamond battery that could last for thousands of years15. UPower is another startup working on a megawatt-scale atomic generator.
Silicon anode
Today, the material typically used for the anode is graphite because it is economical, reliable, and relatively energy-dense, especially compared to current cathode materials. The limiting factor of lithium-ion batteries is the amount of lithium that can be stored in the electrodes. Using silicon as the material for the anode, rather than graphite, allows around nine times more lithium ions to be held in the anode.
The ability to store more lithium ions using silicon sounds amazing; why isn’t everyone doing this? The problem is a silicon anode swells to 3-4 times its original volume when it absorbs lithium ions. Making the casing bigger doesn’t circumvent the problem because the expansion causes the silicon to fracture, causing the battery to fail. It also gums up with a passivation layer, also known as the solid electrolyte interphase (SEI), formed on electrode surfaces from the decomposition of electrolytes.
“With silicon, the cookie crumbles and gets gooey.” - Elon Musk.
As a solution to this problem, many companies use silicon as a fraction of the anode material. But these materials are expensive and highly engineered. Examples of this include silicon structured in SiO glass ($6.6 per kWh), silicon structured in graphite ($10.2 per kWh), and silicon nanowires (>$100 per kWh)16. Sila Nanotechnologies is using silicon as their anode material17. Amprius claims to use silicon for 100% the anode material with silicon nanowires, a highly engineered, expensive material. Advano, Enevate, and Enovix are startups working on a silicon solution for the anode material.
Tesla’s Battery Day
At Tesla’s Battery Day event, they announced many changes to their battery that encompass more than just the materials used. Tesla has on staff one of the most renowned battery scientists, Jeff Dahn. His most recent papers on “A Wide Range of Testing Results on an Excellent Lithium-Ion Cell Chemistry to be used as Benchmarks for New Battery Technologies18” and “Is Cobalt Needed in Ni-rich Positive Electrode Materials for Lithium-Ion Batteries?19” help gives some insight into what Tesla has been working on.
The battery day outcomes increase their vehicles’ range while being more economical; they plan to halve the cost per kilowatt-hour. Most startups20 in this space tend to take a single design decision into account for their products, for example, anode material and focus on that. Tesla, on the other hand, took a very well rounded approach. They took into account not only the materials for the cathode and anode but also the cell design, factory, and integration with the vehicle21.
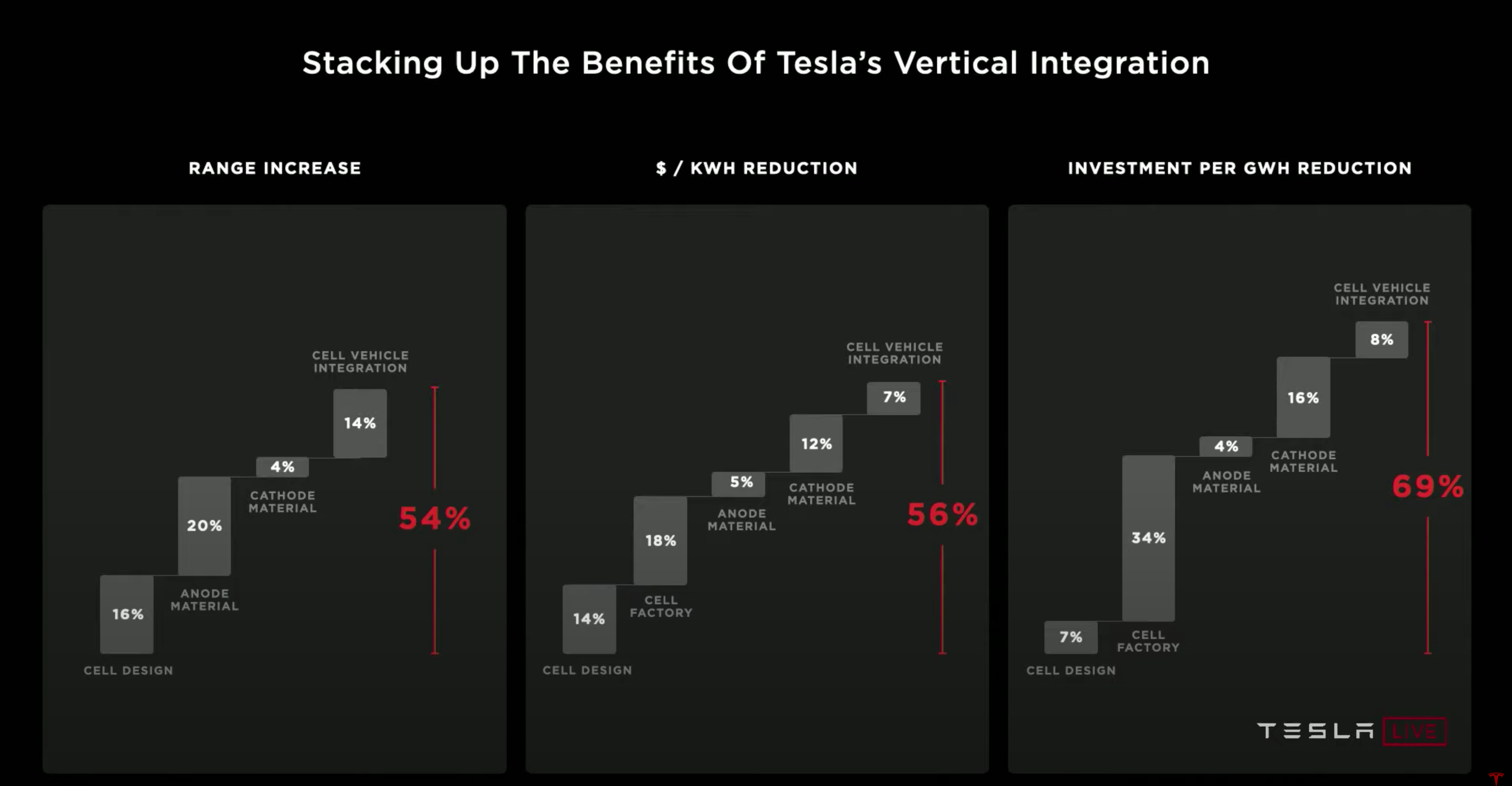
Source: Tesla’s Battery Day Presentation https://www.youtube.com/watch?v=l6T9xIeZTds
Let’s break down each of these improvements.
Cell design
For Tesla’s batteries, while discharging, the positive ions flow over the tabs, while the lithium ions flow from the anode to the cathode, as shown below. The tabs allow the cell’s energy to be transferred to an external source.
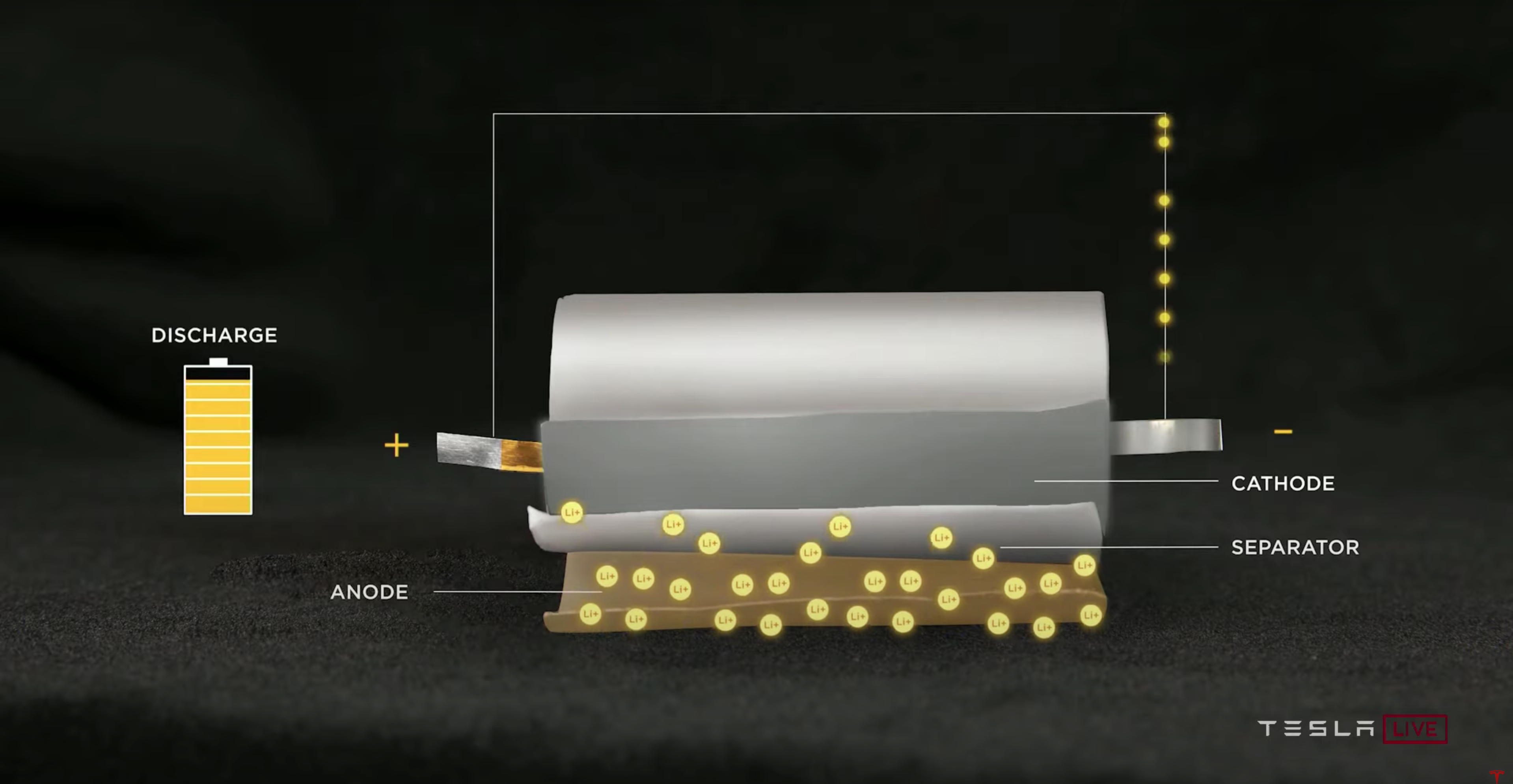
Source: Tesla’s Battery Day Presentation https://www.youtube.com/watch?v=l6T9xIeZTds
The Tesla team sought out to increase the cell size to 46 millimeters, which optimizes vehicle range and cost reduction. However, increasing the cells’ size has a negative side effect on supercharging because of thermal issues. To circumvent these issues, the Tesla team removed the tabs, calling their new design tabless.
The tabless design leads to simpler manufacturing, fewer parts, and a five times reduction in the electrical path. Going from 250-millimeter to 50-millimeter electrical path length leads to substantial thermal benefits. The electrical path length is significant because the distance the electron has to travel is much less. Even though the cell is much bigger, the power to weight ratio is better than a smaller cell with tabs.
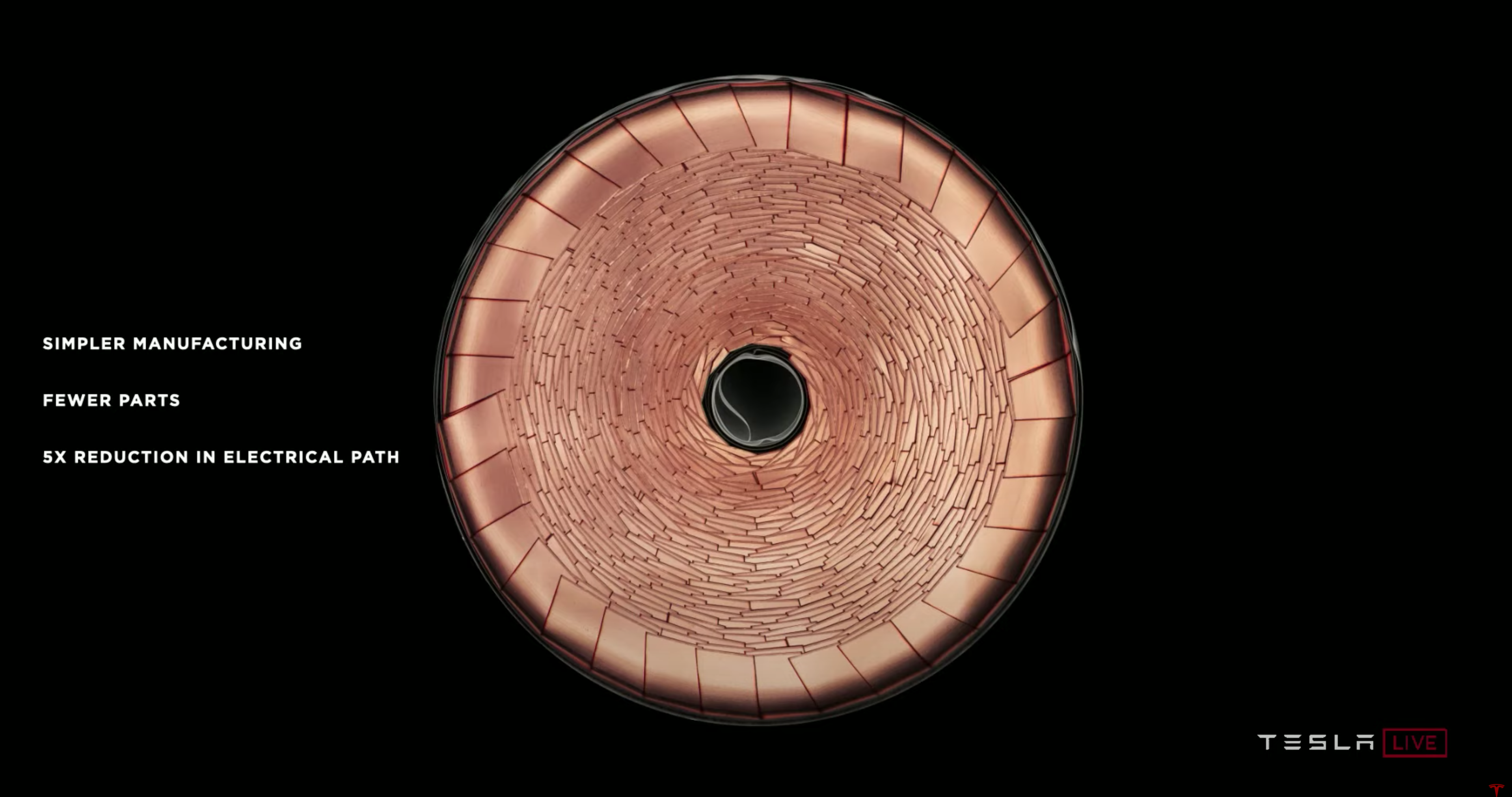
Source: Tesla’s Battery Day Presentation https://www.youtube.com/watch?v=l6T9xIeZTds
Let’s dive into why this new tabless design matters. Instead of calling it tabless, Tesla could have called it “many tabs” because each of the folded pins is a tab, as shown in the image above. What is the function of a tab?
Growing up, my family would always leave sporting events before they ended to avoid the crowd trying to leave the stadium after the event was over. If we had stayed to the end of the event, it would take more time for us to exit the stadium and be very uncomfortable since everyone would be trying to leave through very few exits at the same time. As people are trying to exit, they get closer and closer to one another, and the environment becomes very hot and rowdy. If we think of people as electrons, a stadium with a single exit is similar to a battery’s behavior with a single tab; electrons are all trying to leave through the single tab and bumping up against one another until they heat up. There are multiple tabs in Tesla’s new design, equivalent to a stadium with lots of exits. Now people, or electrons, can exit quickly while staying cool and calm.
There aren’t many details from the presentation on the new tabless design and its implementation, but it can be attributed to “secret sauce.”
Manufacturing a cell consists of an electrode process where the active materials are coated into films onto foils; the coated foils are then wound in the winding process. The roll is then assembled into the can, sealed, and filled with electrolyte and then sent to Formation where the cell is charged for the first time. If you recall from above, a lithium-ion battery starts its life in a discharged state. For a battery cell with tabs, manufacturing is much more complicated. When the cell with tabs is going through the assembly line, it has to keep stopping where all the tabs are so you can’t do continuous motion production. It is also a lot more error-prone.
“It is really a huge pain in the ass to have tabs from a production standpoint.” - Elon Musk.
The new batteries are 46 millimeters by 80 millimeters, leading to the name 4680. The first two digits refer to the diameter, and the second two digits refer to the length. Previously, an extra zero was added onto the end of the name, but it was removed since it had no purpose.
The 4680 batteries have five times more energy with six times the power and enable a 16% range increase. At the battery pack level, the form factor improvements alone lead to a 14% reduction in cost per kWh.
Cell factory
We learned a bit about how removing tabs from the battery cells simplified the manufacturing process above. In an assembly line, you don’t want things to stop and start but continuously move. Any time the process is stopped leads to inefficiency. The Tesla team aims to speed up its process to make one factory have multiple scales of efficiency better than a typical battery factory.
We learned above that the electrode process is where the active materials are coated into films onto foils. The wet process step of the electrode process consists of first: mixing. Mixing occurs when the powders are mixed with either water or a solvent, typically a solvent for the cathode. The mix then goes into a large coat and dry oven, tens of meters long, where the slurry is coated onto the foil and dried. The solvent then has to be recovered. Finally, the coated foil is compressed to the final density. This process is complex and inefficient, especially since humans need to transport the mix from the mixing step to the ovens. It is also inefficient due to the need to put the solvent in and then recover it.
One significant change they are making is skipping the solvent step of the electrode coating’s wet process in favor of a dry process. The dry process transforms the powder directly into film. This technology initially stemmed from Tesla’s acquisition of Maxwell at the beginning of 201922. At battery day, Elon mentioned that since the acquisition, they are now on the 4th revision of the equipment that turns powder into film. Elon noted, “there is still a lot of work to do. There is a clear path to success but a ton of work between here and there.” When this process is scaled up, it results in a ten times reduction in footprint and a ten times reduction in energy, and a massive decrease in CapEx investment.
The manufacturing step known as Formation is where the cell is charged for the first time, and the quality of the cell is verified. Formation is typically 25% of the CapEx investment. The Tesla team improved density and cost-effectiveness by using their knowledge from cars and the powerwall charging and discharging. This led to a 86% reduction in Formation CapEx investment per GWh and a 75% reduction in footprint. For a factory that previously output 150 GWh, this translates to that same factory outputting 1 TWh with the more efficient processes. At the battery pack level, this leads to an 18% reduction in cost per kWh.
Anode material
Tesla announced they were moving to silicon as their anode material. Silicon is excellent because it is the most abundant element in the earth’s crust after oxygen. Rather than creating a highly engineered material that would be expensive, Tesla will use the raw silicon found in the earth’s crust and design for it to expand. They will stabilize the silicon’s surface through an elastic, ion-conducting polymer coating and a highly elastic binder and electrolyte.
Tesla’s silicon costs $1.20 per kWh, whereas the solutions we covered earlier cost anywhere from $6 per kWh to upwards of a hundred. Using silicon leads to a 5% reduction in cost per kWh at the battery pack level and a 20% longer range for Tesla vehicles.
Cathode material
A helpful analogy for understanding the cathode is to think of the cathode as a bookshelf. In this case, the lithium ions would be books. The most efficient bookshelf holds the most books while still being stable enough to retain its structure as the books get loaned out and returned.
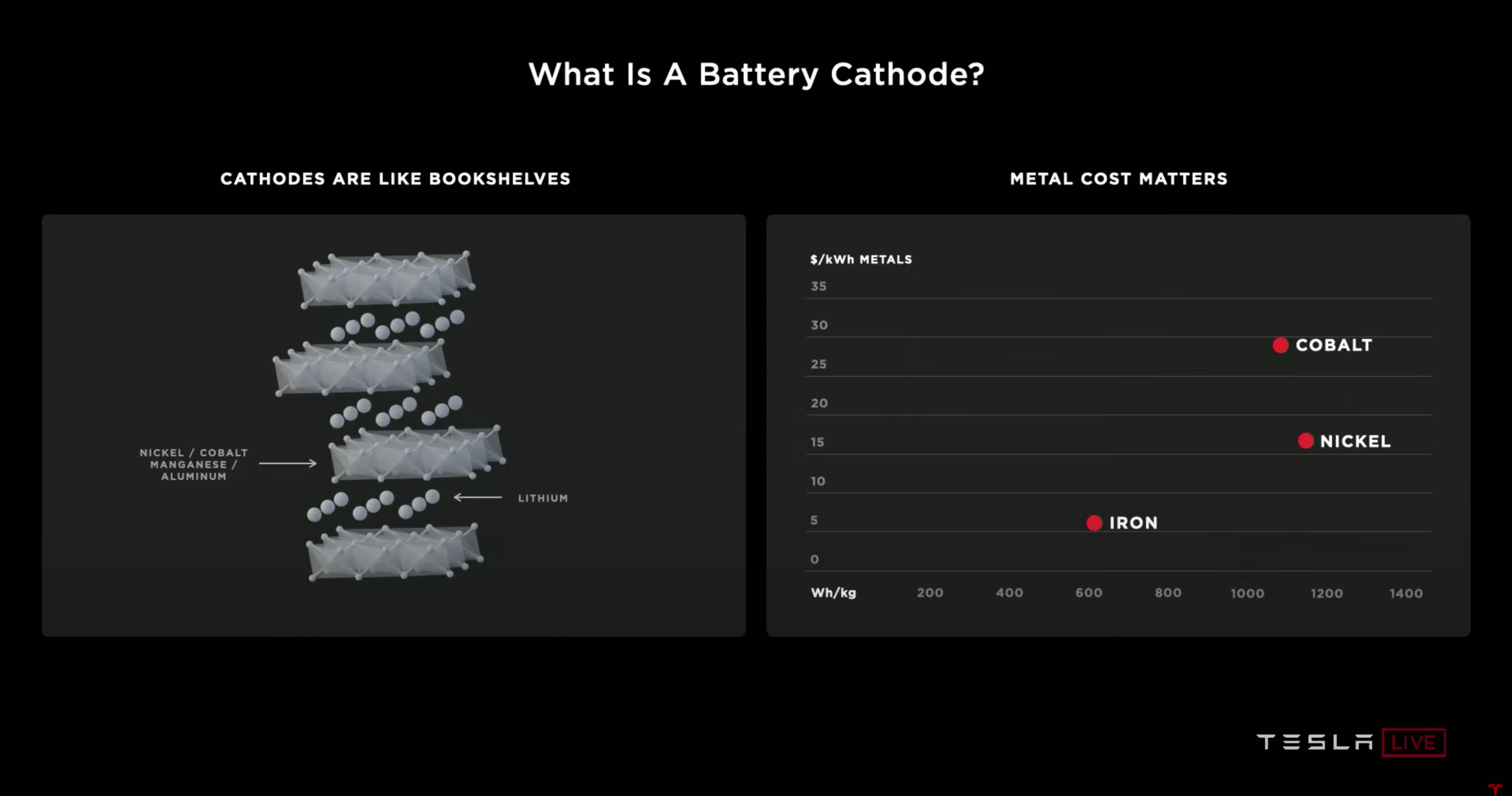
Source: Tesla’s Battery Day Presentation https://www.youtube.com/watch?v=l6T9xIeZTds
The Tesla team aims to increase Nickel in its cathode material since it is the cheapest and has the highest energy density (as shown above). Cobalt is typically used as a cathode material because it is very stable. However, the Tesla team aims to leverage novel coatings and dopants to stabilize Nickel better and remove Cobalt entirely from their materials. Removing Cobalt leads to a 15% reduction in the cathode’s cost per kWh.
The Tesla team made sure to keep in mind the cost of the materials used and the materials’ availability. With silicon for the anode material, availability was not an issue since silicon is readily available. The same goes for lithium, which is also highly accessible. For Nickel, on the other hand, the Tesla team is keeping in mind total Nickel availability by diversifying the amount of Nickel they are using per the type of vehicle.
The team also simplified the cathode manufacturing process by removing all the legacy parts. According to the battery day presentation, the cathode manufacturing process, which is 35% of the cathode cost per kWh, had not had a fresh look in a long time and was wildly inefficient.
“If you take a look at the ‘it’s a small world journey’ of I am a Nickel atom and what happens to me, it’s crazy, you’re going around the world three times, there is a moral equivalent of digging the ditch, filling in the ditch, and digging the ditch again. It’s total madness.” - Elon Musk.
A typical cathode process starts with the metal from the mine being turned into an intermediate material called metal sulfate, which, in turn, is processed again. The Tesla team removed the intermediate step of turning the metal into metal sulfate along with a bunch of other unnecessary steps. They also localized the cathode materials to the US, which decreased the number of miles required for the materials to travel. This leads to a 66% reduction in CapEx investment, a 76% reduction in process cost, and zero wastewater. The cathode material improvements lead to a 12% reduction in cost per kWh at the battery pack level.
Cell vehicle integration
In the early days of aircraft, the fuel was carried as cargo. Later, the fuel tanks were made in wing shape. This was a breakthrough because the wings are critical to the airplane’s function but now could be used for another purpose. The fuel tank was no longer cargo but fundamental to the structure of the aircraft. Tesla intends to do the same for cars.
By removing the intermediate structure in the battery pack, they can pack the cells more densely. Instead of having supports and stabilizers in the battery cells, making up the intermediate structural elements, the battery pack itself is structural. Typically, Tesla fills the battery packs with a flame retardant. The new battery packs will be filled with a flame retardant and structural adhesive, giving it stiffness and stability without intermediate structural elements. This makes the structure even stiffer than a regular car.
The cells can now be moved more towards the center of the vehicle because the volumetric efficiency is better, avoiding a side impact potentially contacting the cells. This also allows the car to maneuver better because the polar moment of inertia is improved. Much like an ice skater can turn better with her arms close to her body rather than extended out.
The improvements to the battery pack integration lead to a 10% mass reduction in the car’s body, a 14% range increase, and 370 fewer parts. The smaller, integrated battery and body also help increase the efficiency of manufacturing. This leads to a 55% reduction in CapEx investment and a 35% reduction in floor space. At the battery pack level, the integration improvements lead to a 7% reduction in cost per kWh.
The sum of all these improvements, including cell design, factory, materials, and vehicle integration, achieves the goal to halve the cost per kWh. Cheaper electric vehicles widen Tesla’s market to new buyers reducing the number of gas-powered vehicles on the road.
Summary
All in all, it is fantastic to see a technology we all rely on day to day get its time in the spotlight. Although not mentioned at battery day, if Tesla were to achieve 400 watt-hours per kilogram, a zero-emissions jet might just be on the horizon. Now that batteries are vertically integrated into Tesla’s product, you can only imagine that the software will track more data on battery efficiency, leading to more and more improvements in the future.
It is incredible to see Tesla take a fresh look at making the most efficient and cost-effective batteries. The level of thought and detail put into rethinking old processes from first principles to make them more efficient is inspiring. The Tesla team didn’t just look at one angle, but all the angles: cell design, manufacturing, vehicle integration, and materials. There is a clear “why” for every decision made that boils down to economics, not just technical gains. Hopefully, we see another core technology, such as batteries, in the spotlight soon.
- https://www.tesla.com/2020shareholdermeeting [return]
- https://www.science.org.au/curious/technology-future/batteries [return]
- https://www.tesla.com/gigafactory [return]
- Some people might, however, be interested in a urine-powered battery: https://newatlas.com/urine-battery/42866/ [return]
- https://time.com/4526350/samsung-galaxy-note-7-recall-problems-overheating-fire/ [return]
- https://qz.com/1588236/how-we-get-to-the-next-big-battery-breakthrough/ [return]
- https://cen.acs.org/articles/95/i46/Solid-state-batteries-inch-way.html [return]
- https://www.caranddriver.com/news/a33435923/toyota-solid-state-battery-2025/ [return]
- https://iopscience.iop.org/nsearch?terms=J%C3%BCrgen+Janek&nextPage=-1&previousPage=-1¤tPage=1&orderBy=relevance&pageLength=10&searchDatePeriod=anytime&journals=1945-7111&authors=J%C3%BCrgen+Janek [return]
- https://www.nature.com/articles/s41560-020-0565-1 [return]
- https://www.nature.com/articles/s41578-019-0157-5 [return]
- https://www.nist.gov/news-events/news/2016/06/physicists-measured-something-new-radioactive-decay-neutrons [return]
- https://phys.org/news/2018-06-prototype-nuclear-battery-power.html [return]
- https://citylabs.net/?option=com_wrapper&view=wrapper&Itemid=20 [return]
- https://techcrunch.com/2020/08/25/self-charging-thousand-year-battery-startup-ndb-aces-key-tests-and-lands-first-beta-customers/ [return]
- https://www.youtube.com/watch?v=l6T9xIeZTds&feature=emb_title [return]
- https://silanano.com/wp-content/uploads/2020/09/The-Future-of-Energy-Storage.pdf [return]
- https://iopscience.iop.org/article/10.1149/2.0981913jes/meta [return]
- https://iopscience.iop.org/article/10.1149/2.1381902jes/meta [return]
- Except for Sila Nanotechnologies, which seems to be most closely aligned with Tesla’s methodology: https://silanano.com/wp-content/uploads/2020/09/The-Future-of-Energy-Storage.pdf [return]
- Tesla claimed in the presentation there were more aspects they didn’t mention they could improve in the future. [return]
- https://electrek.co/2019/02/04/tesla-acquires-ultracapacitor-battery-manufacturer/ [return]